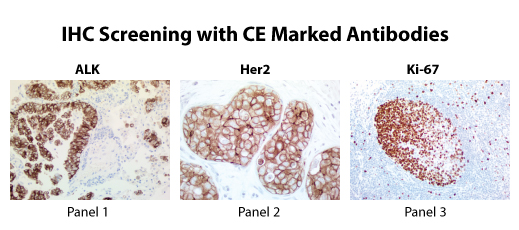
IVD Antibodies
SDIX, OriGene’s wholly owned subsidiary, has manufactured OriGene’s antibodies, including UltraMAB®, in a GMP environment and obtained CE marking for these products. The CE marking provides a level of quality, safety and performance to ensure product reliability and reproducibility. The antibodies are intended for detection of specific protein expression in frozen or formalin fixed human tissues and cells. These antibodies are for in vitro diagnostic (IVD) use*.

* The clinical interpretation of any positive staining or its absence should be complemented by morphological and histological studies with proper controls. Evaluations should be made within the context of the patient's clinical history and other diagnostic tests by a qualified pathologist.
SDIX is now ISO 13485:2003 certified. To learn more about the ISO Certificate and SDIX’s capacity in custom antibody development, please click here.

| 抗体相关资料 |


 United States
United States
 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China
