NME4 (33-187, His-tag) Human Protein
CAT#: AR52009PU-S
NME4 (33-187, His-tag) human protein, 10 µg
Size: 50 ug
|
Need it in bulk or customized? Get a free quote |
CNY 6,900.00
货期*
详询
规格
Specifications
| Product Data | |
| Species | Human |
| Expression Host | E. coli |
| Expression cDNA Clone or AA Sequence |
MGSSHHHHHH SSGLVPRGSH MPSWTRERTL VAVKPDGVQR RLVGDVIQRF ERRGFTLVGM KMLQAPESVL AEHYQDLRRK PFYPALIRYM SSGPVVAMVW EGYNVVRASR AMIGHTDSAE AAPGTIRGDF SVHISRNVIH ASDSVEGAQR EIQLWFQSSE LVSWADGGQH SSIHPA
|
| Tag | His-tag |
| Predicted MW | 19.6 kDa |
| Concentration | lot specific |
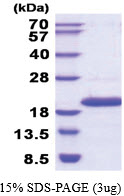
| Purity | >90% by SDS - PAGE |
| Buffer | Presentation State: Purified State: Liquid purified protein Buffer System: 20 mM Tris-HCl buffer (pH 8.0) containing 40% glycerol, 0.2M NaCl |
| Preparation | Liquid purified protein |
| Storage | Store undiluted at 2-8°C for one week or (in aliquots) at -20°C to -80°C for longer. Avoid repeated freezing and thawing. |
| Stability | Shelf life: one year from despatch. |
| Bioactivity | Specific: Specific activity is > 120 units/mg, and is defined as the amount of enzyme that convert 1.0 umole each of ATP and TDP to ADP and TTP per minute at pH 7.5 at 25C in a couple system with PK/LDH. |
| Reference Data | |
| RefSeq | NP_001273362 |
| Locus ID | 4833 |
| UniProt ID | O00746, A0A087WVT9 |
| Cytogenetics | 16p13.3 |
| Synonyms | NDPK-D; nm23-H4; NM23H4 |
| Summary | The nucleoside diphosphate (NDP) kinases (EC 2.7.4.6) are ubiquitous enzymes that catalyze transfer of gamma-phosphates, via a phosphohistidine intermediate, between nucleoside and dioxynucleoside tri- and diphosphates. The enzymes are products of the nm23 gene family, which includes NME4 (Milon et al., 1997 [PubMed 9099850]).[supplied by OMIM, May 2008] |
| Protein Families | Druggable Genome |
| Protein Pathways | Metabolic pathways, Purine metabolism, Pyrimidine metabolism |
Documents
| FAQs |
| SDS |
Customer
Reviews
Loading...


 United States
United States
 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China